Are Noble Gases Very Reactive
Group 18: Reactions of Nobel Gases
- Page ID
- 35711
The noble gases (Group 18) are located in the far right of the periodic table and were previously referred to every bit the "inert gases" due to the fact that their filled valence shells (octets) brand them extremely nonreactive.
The Chemical Properties
Noble gases are odorless, colorless, nonflammable, and monotonic gases that have depression chemical reactivity.
| Atomic Number | Chemical element | Number of Electrons/Shell |
|---|---|---|
| two | Helium | 2 |
| 10 | Neon | two,8 |
| eighteen | Argon | 2,8,8 |
| 36 | Krypton | 2,8,18,8 |
| 54 | Xenon | 2,eight,eighteen,18,8 |
| 86 | Radon | 2,8,18,32,18,8 |
The full valence electron shells of these atoms make noble gases extremely stable and unlikely to class chemical bonds considering they take picayune tendency to gain or lose electrons. Although noble gases do not ordinarily react with other elements to form compounds, in that location are some exceptions. Xe may form compounds with fluoride and oxide.
Example 1: Xenon Fluorides
Xenon Difluoride (\(XeF_2\))
- Dense white crystallized solid
- Powerful fluorinating agent
- Covalent inorganic fluorides
- Stable xenon chemical compound
- Decomposes on contact with calorie-free or water vapor
- Linear geometry
- Wet sensitive
- Low vapor force per unit area
Xenon Tetrafluoride (\(XeF_4\))

- Colorless Crystals
- Foursquare planar geometry
- Discovered in 1963
Xenon Hexafluoride (\(XeF_6\))
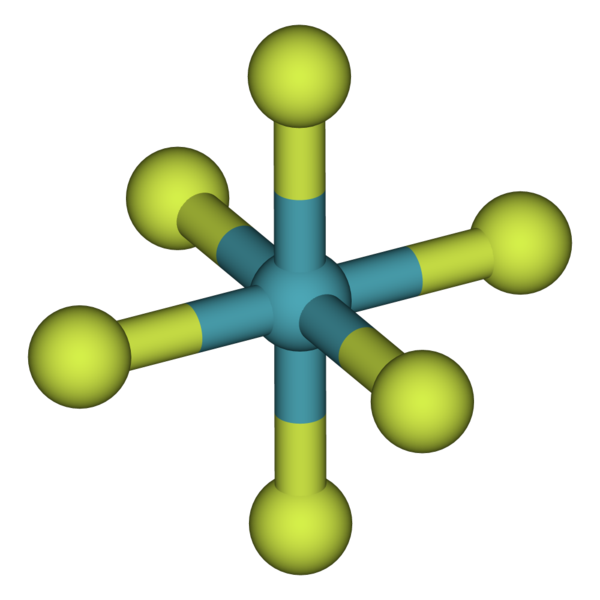
- Strongest fluorinating agent
- Colorless solid
- Highest coordination of the three binary fluorides of xenon (\(XeF_2\) and \(XeF_4\))
- Germination is exergonic, and the compound is stable at normal temperatures
- Readily sublimes into intense xanthous vapors
- Structure lacks perfect octahedral symmetry
Example 2: Xenon Oxide
Xenon Tetroxide (XeO4)
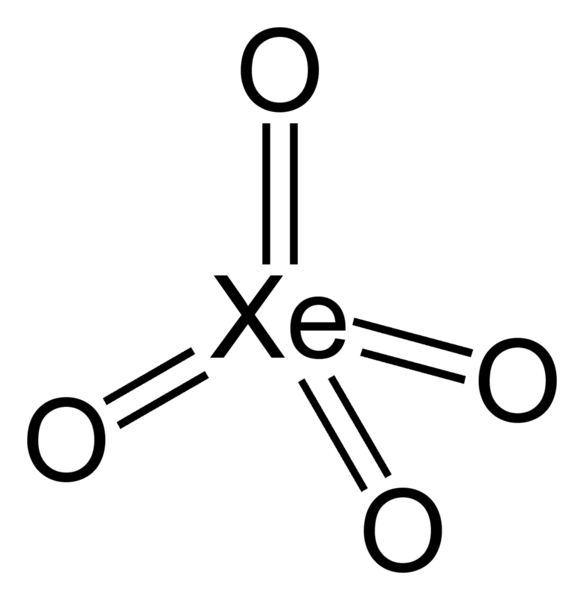
- Yellow crystalline solid
- Relatively stable
- Oxygen is the only element that can bring xenon upward to its highest oxidation state of +8
Two other curt-lived xenon compounds with an oxidation state of +8, XeOthreeF2 and XeO2Fiv, are produced in the reaction of xenon tetroxide with xenon hexafluoride.
Example 3: Radon Compounds
Radon difluoride (RnF2) is one of the few reported compounds of radon. Radon reacts readily with fluorine to form a solid compound, but this decomposes on attempted vaporization and its exact limerick is uncertain. The usefulness of radon compounds is limited because of the element of group 0'southward radioactive decay. The longest-lived isotope, 222Ra, has a half-life of merely 3.82 days.
Are Noble Gases Very Reactive,
Source: https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_and_Websites_(Inorganic_Chemistry)/Descriptive_Chemistry/Elements_Organized_by_Block/2_p-Block_Elements/Group_18%3A_The_Noble_Gases/2Group_18%3A_Reactions_of_Nobel_Gases
Posted by: tusseyfalf1986.blogspot.com


0 Response to "Are Noble Gases Very Reactive"
Post a Comment